Welcome to Tech Observer,
Since 2005, we have proudly served as the preferred partners in clinical research for a diverse range of clients—ranging from multinational corporations to boutique healthcare innovators—across all phases of clinical trials. Despite the diversity of our partnerships and experiences, our unwavering commitment to quality and innovation has remained at the core of everything we do.
Your Success is our priority
We uphold a robust governance structure designed to ensure accountability, accelerate decision-making, and minimize risk at every stage of your research journey.
We have established effective leadership frameworks spanning strategic, operational, and tactical touchpoints to provide clear oversight and drive optimal results for your project, regardless of its size, timeline, or budget. Our streamlined escalation process ensures swift communication across all levels, enabling prompt resolution of any challenges encountered along the way.

Our Clinical Services
Our BioPharma Innovations Services (BIS)
Leaders in Clinical Research
150+ Studies, 1800+ Sites Initiated, 120,000 Subjects Recruited
200+ Database Locks, 1300+ Active Users in 17 Countries, Oracle | INFORM | RAVE | Veeva MedDRA | WHODD
150+ Analysis Report | SAS Software
510+ Medical Writing Assignments, 120+ Clinical Study, Reports, 50+ Study Protocols, 120+ Manuscripts Published in Peer review journals
Areas of Expertise
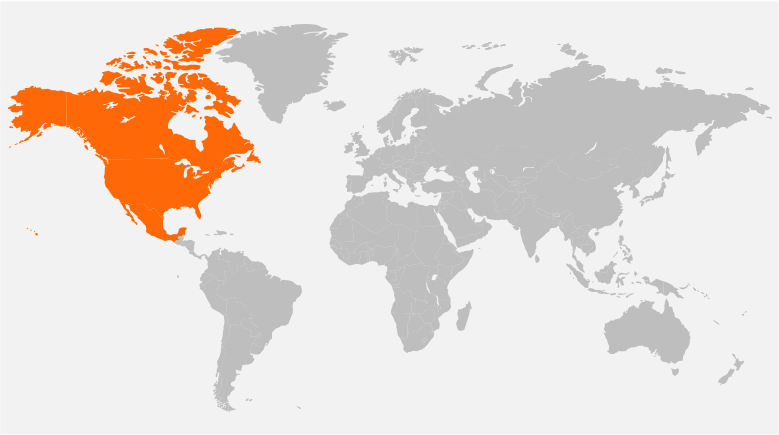
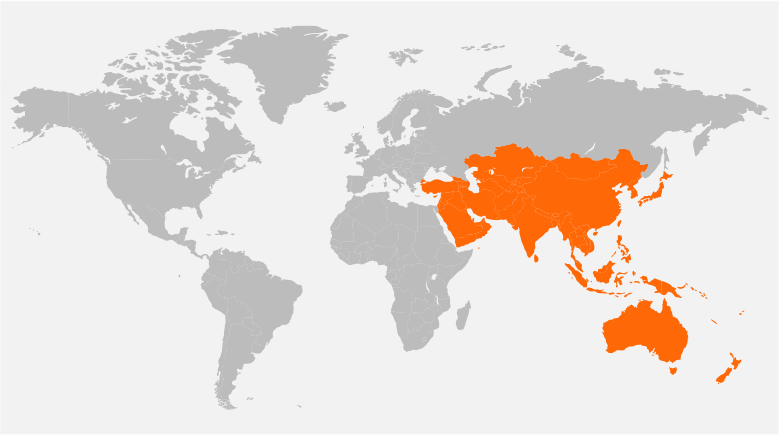
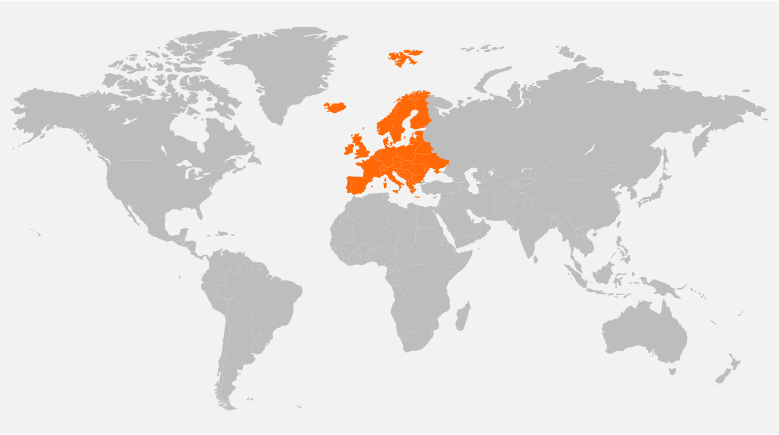
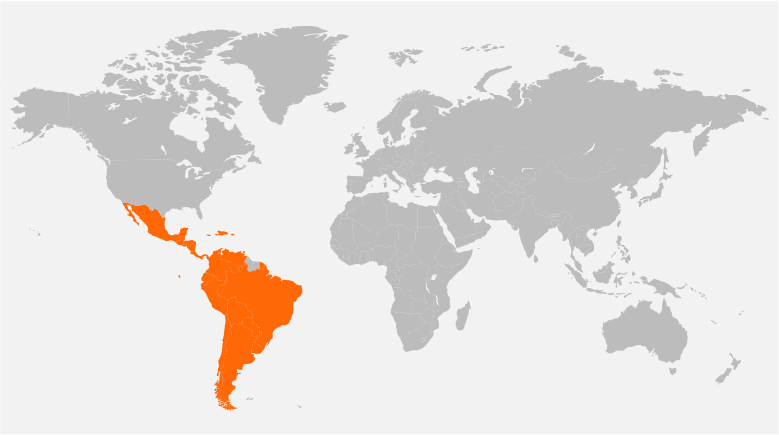
Our Global Reach
Headquartered in New Jersey, USA, and with offices in Singapore, Denmark, United Kingdom, and India, Tech Observer has the reach and experience to support clinical research pipeline worldwide.
Our strategic global presence offers flexibility, local and cultural understanding to manage every trial for the best outcome; ensuring success, discretion, and compassion in the journey of every clinical subject.
Unites States, Canada, Mexico
Australia, Hong Kong, India, Indonesia, Korea, Malaysia, Philippines, Russia, Singapore, Thailand, Taiwan, Vietnam, Turkey, Japan
Denmark, Spain, United Kingdom, Switzerland, France, Netherland, Armenia, Belgium, Germany, Ireland, Italy, Sweden, Poland
Brazil, Argentina, Colombia, Chile
Egypt, South Africa, UAE